No difference in overall incidence of adverse events (AEs) vs. placebo.1,2

of AEs were mild or moderate1,2

Most AEs occurred within the first 3 months2
Most AEs resolved within 2 weeks2
In the GLOW study, more than 1 out of 3 patients did experience GI AEs, so it is important to manage expectations with your patients.1
Here are two ways to learn more about Plenity:
Overview of treatment-related adverse events.1-3
The overall incidence of of all AEs in the Plenity group was no different than the placebo group.1,2
| Parameter | Plenity (n=223) | Placebo (n=211) | Difference (95% CI)² | P value² | |||
|---|---|---|---|---|---|---|---|
| Related AEs | 39.5% | (88) | 30.3% | (64) | 9.1 | (-0.2, 18.2) | 0.06 |
| Eye disorders | 0% | (0) | 0.5% | (1) | -0.5 | (-3.0, 1.7) | 0.49 |
| GI disorders | 37.7% | (84) | 27.5% | (58) | 10.2 | (1.0, 19.1) | 0.02 |
| General disorders | 0.4% | (1) | 0.5% | (1) | -0.0 | (-2.6, 2.4) | 1.00 |
| Infection/infestations | 0.9% | (2) | 0.5% | (1) | 0.4 | (-2.2, 3.1) | 1.00 |
| Investigations | 1.3% | (3) | 1.4% | (3) | -0.1 | (-3.3, 3.0) | 1.00 |
| Metabolism/nutrition | 0% | (0) | 1.9% | (4) | -1.9 | (-5.1, 0.6) | 0.06 |
| MSK/connective tissue | 0.9% | (2) | 0% | (0) | 0.9 | (-1.5, 3.5) | 0.50 |
| Nervous system | 1.8% | (4) | 0.9% | (2) | 0.8 | (-2.2, 4.0) | 0.69 |
| Renal/urinary | 0.4% | (1) | 0% | (0) | 0.4 | (-1.8, 2.9) | 1.00 |
| Reproductive | 0% | (0) | 0.5% | (1) | -0.5 | (-3.0, 1.7) | 0.49 |
| Respiratory/thoracic | 0.4% | (1) | 0.5% | (1) | -0.0 | (-2.6, 2.4) | 1.00 |
| Skin/subcutaneos | 0.4% | (1) | 1.4% | (3) | -1.0 | (-4,0, 1.7) | 0.36 |
| The dropout rate was lower in the Plenity group (22%) than the placebo group (29%)2 Only the category of GI disorders related to Plenity was different relative to Placebo (p=0.02488).1,2 |
Treatment-related GI AEs as assessed by investigators.1,2
| Parameter | Plenity % (n) | Placebo % (n) |
|---|---|---|
| GI-related AEs* | 37.7% (84) | 27.5% (58) |
| Abdominal distension | 10.8% (24) | 5.7% (12) |
| Diarrhea | 10.3% (23) | 7.6% (16) |
| Infrequent bowel movements | 9.0% (20) | 4.7% (10) |
| Flatulence | 8.5% (19) | 4.7% (10) |
| Abdominal pain | 4.9% (11) | 2.8% (6) |
| Constipation | 4.5% (10) | 4.7% (10) |
| The most common (>5%) GI AEs in the Plenity group were diarrhea, abdominal distension, infrequent bowel movements, flatulence, constipation, abdominal pain, and nausea.1,2 75% (119 out of 158) of GI AEs in the Plenity group and 79% (83 of 105) in the placebo group were assessed as mild.1 |
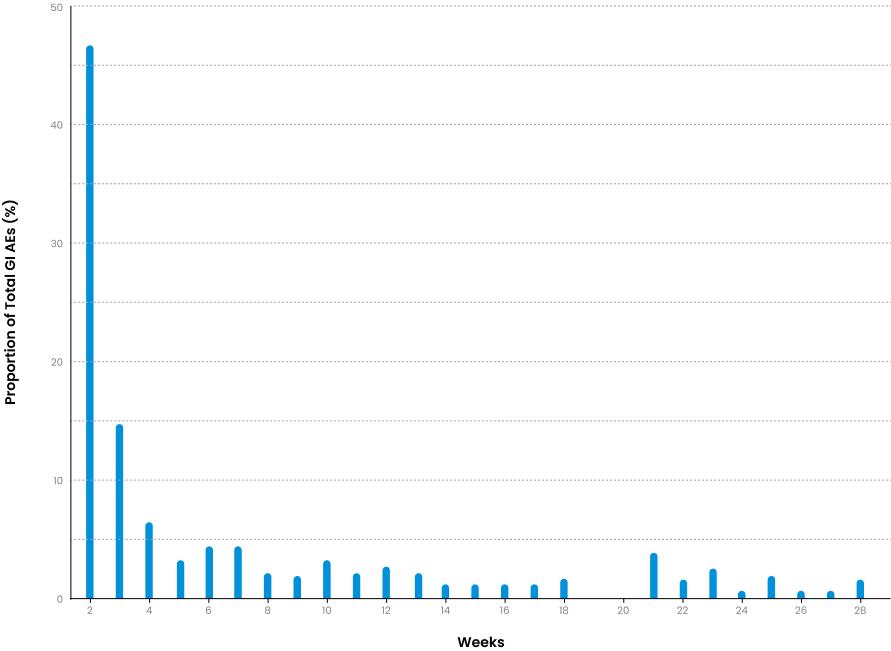
Incidence of treatment-related GI AEs.
In the trial, 37.7% of Plenity patients experienced GI-related AEs - most occurred within the first 2 week and the majority resolved within 2 weeks of onset.2,4
Note: Plenity data only (not relative to placebo).
Intended Use
Plenity® is indicated to aid weight management in adults with excess weight or obesity, a Body Mass Index (BMI) of 25–40 kg/m², when used in conjunction with diet and exercise.
Important Safety Information
- Plenity is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin, or titanium dioxide
- Plenity may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for Use carefully
- Avoid use in patients with: esophageal anatomic anomalies, including webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); and complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility
- Use with caution in patients with active gastrointestinal conditions such as gastro-esophageal reflux disease (GERD), ulcers, or heartburn
- The overall incidence of AEs in the Plenity group was no different than the placebo group
- The most common side effects were diarrhea, distended abdomen, infrequent bowel movements, and flatulence
Rx Only. For the safe and proper use of Plenity, refer to the Healthcare Professionals Instructions for Use.
- Plenity (Instructions for Use). Boston, MA: Gelesis, Inc.; 2021.
- Greenway FL, Aronne LJ, RabenA, et al. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemicoral hydrogel for weight loss. Obesity. 2019;27(2):205–216.
- Data on file. Gelesis, Inc.
- Luzi L, Raben A, Astrup A, et al. Comprehensive analysis of safety and tolerability of Gelesis100 in overweight and obesity in the pivotal GLOW study. Poster presented at: European and International Congress on Obesity; September 1–4, 2020; virtual meeting.
AE=adverse event; CI=confidence interval; GI=gastrointestinal; MSK=musculoskeletal



