The Gelesis Loss of Weight (GLOW) study
IN GLOW, A 6-MONTH TRIAL
~6 out of 10 adult responders achieved a ≥5% weight loss.*,2
Those 6 out of 10 lost an average of:1,2
- ~10% of total body weight1,2
- ~22 lbs1,2
*The GLOW pivotal study was a 6-month, multicenter, randomized, double-blind, placebo-controlled pivotal trial assessing the safety and efficacy of Plenity. Plenity (n=223) or placebo (n=213) was administered to 436 adults with excess weight or obesity, with or without type 2 diabetes. Primary endpoints: 1) at least 35% of patients on Plenity achieving ≥5% weight loss, and 2) whether individuals receiving Plenity lost 3% more of their body weight than individuals receiving placebo.1
IN GLOW, A 6-MONTH TRIAL
1 in 4 adults were super responders who achieved ≥10% weight loss.*,2
Those 1 in 4 lost an average of:1-3
- ~14% of total body weight1-3
- ~30 lbs1-3
*The GLOW pivotal study was a 6-month, multicenter, randomized, double-blind, placebo-controlled pivotal trial assessing the safety and efficacy of Plenity. Plenity (n=223) or placebo (n=213) was administered to 436 adults with excess weight or obesity, with or without type 2 diabetes. Primary endpoints: 1) at least 35% of patients on Plenity achieving ≥5% weight loss, and 2) whether individuals receiving Plenity lost 3% more of their body weight than individuals receiving placebo.1

Here are two ways to learn more about Plenity:
Study Design
GLOW Study
The Gelesis Loss of Weight (GLOW) study was a 6-month, multicenter, randomized, double-blind, placebo-controlled pivotal trial assessing the safety and efficacy of Plenity.
Plenity (n=223) or Placebo (n=213) was administered to 436 adults with excess weight or obesity, with or without type 2 diabetes. Patients were randomized to 2.25 g of Plenity or placebo and were prescribed reduced caloric intake and exercise. The co-primary endpoints were: 1) at least 35% of patients on Plenity achieving ≥5% weight loss, and 2) whether individuals receiving Plenity lost 3% more of their body weight than individuals receiving placebo.
The study met and exceeded the categorical endpoint. It did not achieve super-superiority. The percentage of patients who lost ≥5% of body weight was 59%. Individuals on Plenity lost on average 6% vs. individuals on placebo who lost on average 4% (P=0.0007).1
There was a clear and early separation between responders and non-responders, which may allow for an early prediction of response to therapy. More specifically, in this post-hoc analysis, weight loss of at least 3% as early as after 8 weeks of treatment predicted clinically meaningful weight loss at 6 months, with sensitivity and specificity levels exceeding 80%.
GLOW key inclusion criteria1-3
- Male or female ambulatory patients
- Aged ≥22 and ≤65 years
- BMI ≥27 and ≤40 kg/m2
- Patients with BMI <30 kg/m2 with ≥1 comorbidity:
- Untreated or metformin treated T2D
- Untreated or drug-treated dyslipidemia
- Untreated or drug-treated hypertension
- FPG ≥90 and ≤145 mg/dL
GLOW key exclusion criteria2,3
- Contraception cessation2
- Pregnancy1
- Type 1 diabetes1,2
- HbA1c >8.5% (>69 mmol/L)2,3
- Serum LDL-C ≥190 mg/dL or TG ≥500mg/dL3
- Needed to take medications at lunch and dinner2,3
- History of eating disorders2
- Stopped smoking within 6 months3
- Had weight change >3 kg within 3 months prior to or during screening2,3
- History of GI or endocrine disease or major GI disorders1,2
- History of gastric bypass or other surgeries in the GI system2
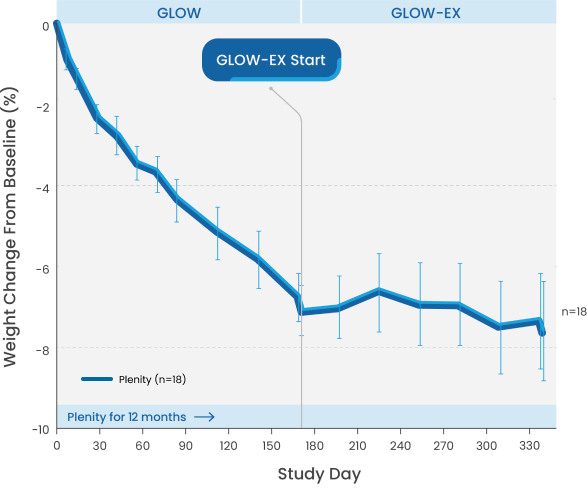
GLOW-EX, a 6-month extension of the pivotal trial²
Sustained weight loss demonstrated to 1 year.1-2
Patients initially treated with Plenity who continued with treatment successfully maintained their weight loss for a total of 12 months.
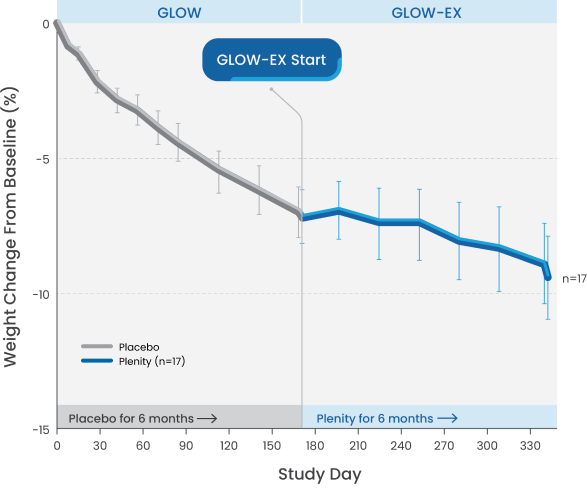
Additional weight loss with Plenity.1,2
Patients initially treated with placebo and switched to Plenity achieved incremental weight loss and maintained it for an additional 6 months.
GLOW-EX study design
GLOW-EX was a 6-month open-label extension study for adults who completed treatment (placebo or Plenity) and lost ≥3% of their body weight from baseline (N=39). The objective of the study was to evaluate the safety of long-term exposure (1 year) to Plenity and the effectiveness of Plenity in maintaining weight loss achieved after 6 months (combined with lifestyle modification).1,2
In a patient survey conducted in a real-world setting, a majority of responders:*
agreed or strongly agreed that Plenity helped them feel less hungry (n=40)3
reported feeling somewhat satisfied or satisfied after meals (n=38)3
stated Plenity started working for them within the first 4 weeks (n=36)3
Survey overview3
- This was a market research activity conducted to obtain patient experience with Plenity
- Adults (n=166) with a BMI of 25 to 40kg/m² participated in a 6-month program
- Eligible participants were required to have self-reported success losing 10 lbs with an alternate weight loss method
- Initial HCP appointments were conducted virtually, and eligible participants received Plenity by mail at no cost for the program span
- Participants completed a baseline questionnaire and a non-validated, close-ended questionnaire at the end of the 6-month program
- A total of 137 participants (83%) successfully completed the 6-month program, of which 51 participants (37%) completed the voluntary end-of-program survey
*Unpublished data from the Plenity Invitation Only Program. All results are self-reported. Data do not reflect full participant sample. Represents responses at week 30 (n=51).3
Ready to prescribe?
Intended Use
Plenity® is indicated to aid weight management in adults with excess weight or obesity, a Body Mass Index (BMI) of 25–40 kg/m², when used in conjunction with diet and exercise.
Important Safety Information
- Plenity is contraindicated in patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin, or titanium dioxide
- Plenity may alter the absorption of medications. Read Sections 6 and 8.3 of the Instructions for Use carefully
- Avoid use in patients with: esophageal anatomic anomalies, including webs, diverticuli, and rings; suspected strictures (such as patients with Crohn’s disease); and complications from prior gastrointestinal (GI) surgery that could affect GI transit and motility
- Use with caution in patients with active gastrointestinal conditions such as gastro-esophageal reflux disease (GERD), ulcers, or heartburn
- The overall incidence of AEs in the Plenity group was no different than the placebo group
- The most common side effects were diarrhea, distended abdomen, infrequent bowel movements, and flatulence
Rx Only. For the safe and proper use of Plenity, refer to the Healthcare Professionals Instructions for Use.
- Plenity (Instructions for Use). Boston, MA: Gelesis, Inc.; 2021.
- Greenway FL, Aronne LJ, RabenA, et al. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemicoral hydrogel for weight loss. Obesity. 2019;27(2):205–216.
- Data on file. Gelesis, Inc.
BMI=body mass index; FPG=fasting plasma glucose; GI=gastrointestinal; HCP=healthcare provider; HbA1c=hemoglobin A1c; LDL-C=low-density lipoprotein cholesterol; T2D=type 2 diabetes; TG=triglycerides.



